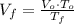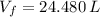Chemistry, 02.03.2021 14:00 kiingbr335yoqzaxs
A balloon filled with helium has a volume of 30.0 L at a pressure of 100 kPa and a temperature of 15.0 C. What will the volume of the balloon be if the temperature is increased to 80.0 C and the pressure remains constant? (Hint: Use the Charles law equation)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1


Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
A balloon filled with helium has a volume of 30.0 L at a pressure of 100 kPa and a temperature of 15...
Questions




Social Studies, 17.01.2020 00:31

Mathematics, 17.01.2020 00:31


Mathematics, 17.01.2020 00:31

English, 17.01.2020 00:31

History, 17.01.2020 00:31

Social Studies, 17.01.2020 00:31




Biology, 17.01.2020 00:31





Mathematics, 17.01.2020 00:31

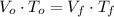 (1)
(1)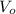 ,
, 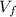 - Initial and final volume, measured in liters.
- Initial and final volume, measured in liters.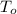 ,
, 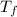 - Initial and final temperature, measured in Kelvin.
- Initial and final temperature, measured in Kelvin.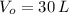 ,
, 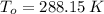 and
and 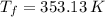 , the final volume of the balloon is:
, the final volume of the balloon is: