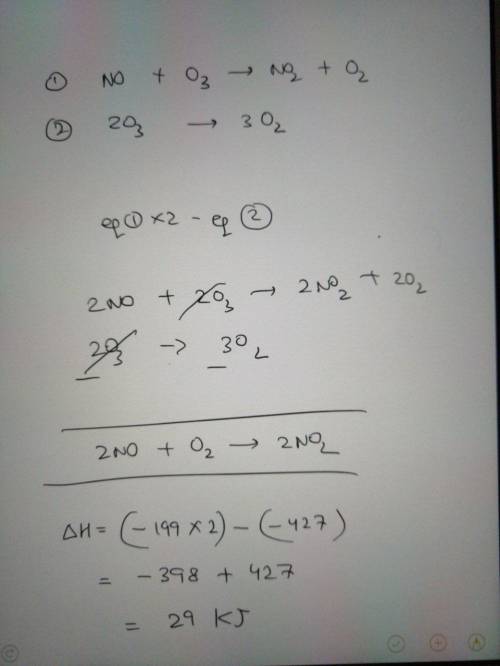Nitric oxide gas, NO(g), can be oxidized in air to
produce nitrogen dioxide gas, NO2(g):
2 NO...

Chemistry, 02.03.2021 14:40 dinosaur10
Nitric oxide gas, NO(g), can be oxidized in air to
produce nitrogen dioxide gas, NO2(g):
2 NO(g) + O2(g) → 2 NO2(g)
Determine the enthalpy change for this reaction
using any of these thermochemical equations:
02(g) →20(g)
AH = +495 kJ

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

You know the right answer?
Questions

Mathematics, 16.12.2020 21:50

Mathematics, 16.12.2020 21:50



Mathematics, 16.12.2020 21:50

Chemistry, 16.12.2020 21:50


Mathematics, 16.12.2020 21:50


Health, 16.12.2020 21:50

Mathematics, 16.12.2020 21:50

Mathematics, 16.12.2020 21:50


Mathematics, 16.12.2020 21:50


Mathematics, 16.12.2020 21:50


Biology, 16.12.2020 21:50

English, 16.12.2020 21:50

Mathematics, 16.12.2020 21:50