
Chemistry, 02.03.2021 22:00 carolinehodges
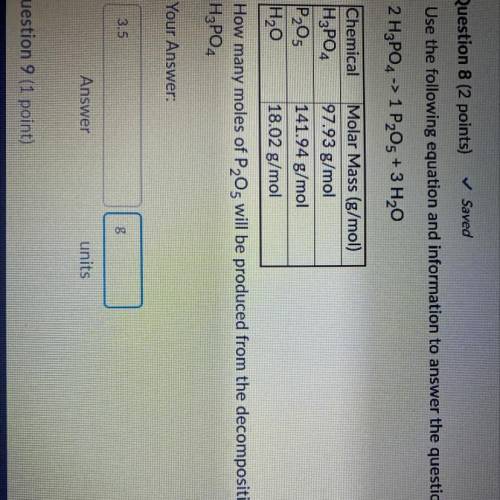
Use the following equation and information to answer the question that follows
2 H3PO4 -> 1 P205 + 3 H20
Chemical
Molar Mass (g/mol)
| H₂PO4 97.93 g/mol
P₂O5 141.94 g/mol
H2O 18.02 g/mol
How many moles of P2O5 will be produced from the decomposition of 0.833 mol of
H3PO4

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

You know the right answer?
Use the following equation and information to answer the question that follows
2 H3PO4 -> 1 P205...
Questions

Mathematics, 10.12.2020 02:10



Mathematics, 10.12.2020 02:10



Mathematics, 10.12.2020 02:10


Chemistry, 10.12.2020 02:10



Mathematics, 10.12.2020 02:10

Mathematics, 10.12.2020 02:10






Health, 10.12.2020 02:10

Health, 10.12.2020 02:10



