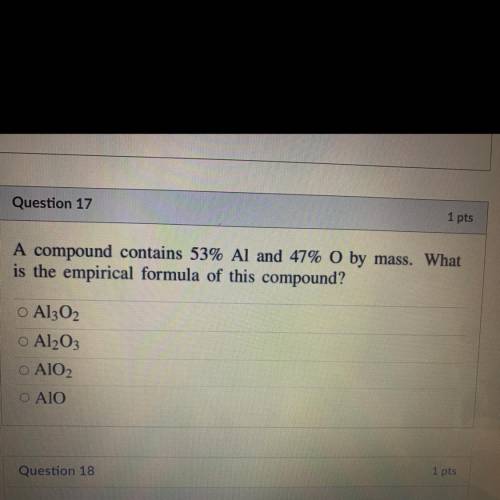
A compound contains 53% Al and 47% O by mass. What
is the empirical formula of this compound?...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1
You know the right answer?
Questions


Mathematics, 30.10.2019 00:31

Mathematics, 30.10.2019 00:31


Mathematics, 30.10.2019 00:31

Mathematics, 30.10.2019 00:31

Mathematics, 30.10.2019 00:31



Health, 30.10.2019 00:31


Mathematics, 30.10.2019 00:31


English, 30.10.2019 00:31

Health, 30.10.2019 00:31





Mathematics, 30.10.2019 00:31