
Chemistry, 02.03.2021 23:40 MalikaJones
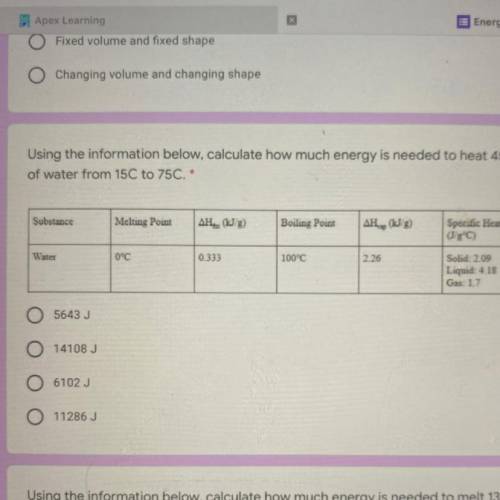
Using the information below, calculate how much energy is needed to heat 45 g
of water from 15C to 75C. *
1. 5643 J
2. 14108 J
3. 6102 J
4. 11286J

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 12:30
If you reacted 450 g of trimethylgallium with 300 g of arsine, what mass of gaas could you make?
Answers: 1

Chemistry, 23.06.2019 18:10
Which is an aspect of the kinetic-molecular theory and can be used to explain the behavior of plasmas? particle spacing can allow a very high density. particle kinetic energy is independent of temperature. particles vibrate quickly in stationary positions. particles exchange energy through elastic collisions.
Answers: 2
You know the right answer?
Using the information below, calculate how much energy is needed to heat 45 g
of water from 15C to...
Questions


Social Studies, 06.10.2019 10:30


Mathematics, 06.10.2019 10:30


Biology, 06.10.2019 10:30

History, 06.10.2019 10:30

Biology, 06.10.2019 10:30

Mathematics, 06.10.2019 10:30


Mathematics, 06.10.2019 10:30











