Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
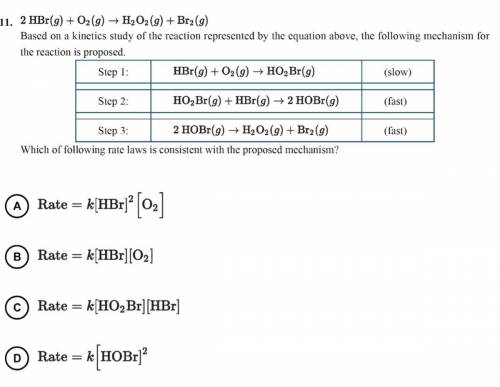
2 HBr(g)+O2(g)—>H2O2(g)+Br2(g)
Based on a kinetics study of the reaction represented by the equ...
Questions

Advanced Placement (AP), 19.03.2021 02:30




Mathematics, 19.03.2021 02:30



Mathematics, 19.03.2021 02:30


Mathematics, 19.03.2021 02:30







Mathematics, 19.03.2021 02:30


History, 19.03.2021 02:30

Mathematics, 19.03.2021 02:30