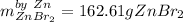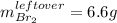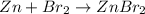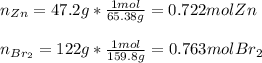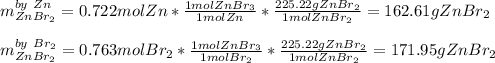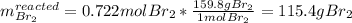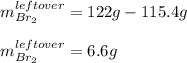Chemistry, 03.03.2021 08:40 parkermacyow71bm
A. Which reactant is the limiting reagent?
b. How much product is formed? (calculate for both if 2)
c. How much of the excess reagent remains?
1) 47.2 g of zinc metal react with 122 g of bromine in a closed container,
Final answers
la.
1b.
1c.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

You know the right answer?
A. Which reactant is the limiting reagent?
b. How much product is formed? (calculate for both if 2)...
Questions

Mathematics, 26.03.2021 03:40


Mathematics, 26.03.2021 03:40




Social Studies, 26.03.2021 03:40


Mathematics, 26.03.2021 03:40

Mathematics, 26.03.2021 03:40

Mathematics, 26.03.2021 03:40

Mathematics, 26.03.2021 03:40

Health, 26.03.2021 03:40

Mathematics, 26.03.2021 03:40

Medicine, 26.03.2021 03:40



Mathematics, 26.03.2021 03:40

Mathematics, 26.03.2021 03:40