

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2


Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3
You know the right answer?
For the reaction shown, calculate how many grams of oxygen form when each quantity of reactant compl...
Questions




Social Studies, 03.07.2019 17:00


Chemistry, 03.07.2019 17:00


History, 03.07.2019 17:00



Social Studies, 03.07.2019 17:00

Advanced Placement (AP), 03.07.2019 17:00

Chemistry, 03.07.2019 17:00

Mathematics, 03.07.2019 17:00

History, 03.07.2019 17:00

Mathematics, 03.07.2019 17:00



Biology, 03.07.2019 17:00

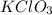 is 1.06 grams.
is 1.06 grams.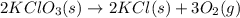
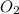 = 32 g/mol
= 32 g/mol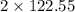 grams of
grams of 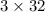 grams of
grams of 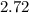 grams of
grams of 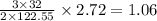 grams of
grams of 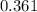 grams of
grams of 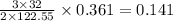 grams of
grams of  of
of 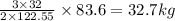 of
of  of
of 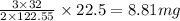 of
of 

