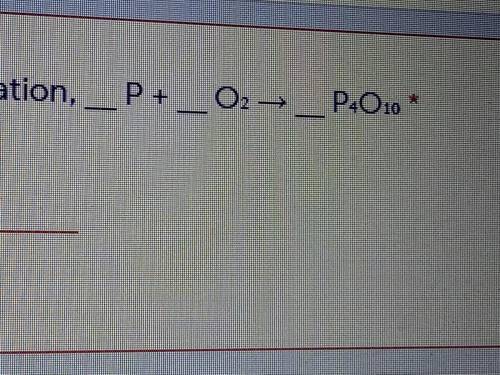
Fill in the blanks to balance equation __P+__O2 ---> __P4 O10
...

Chemistry, 03.03.2021 19:50 chandlergward8
Fill in the blanks to balance equation __P+__O2 ---> __P4 O10

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1


Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
Questions


Mathematics, 12.03.2021 07:00

Biology, 12.03.2021 07:00

Mathematics, 12.03.2021 07:00

Social Studies, 12.03.2021 07:00

English, 12.03.2021 07:00


Mathematics, 12.03.2021 07:00


History, 12.03.2021 07:00

History, 12.03.2021 07:00


Social Studies, 12.03.2021 07:00

Chemistry, 12.03.2021 07:00

Physics, 12.03.2021 07:00

Mathematics, 12.03.2021 07:00

Social Studies, 12.03.2021 07:00



Mathematics, 12.03.2021 07:00



