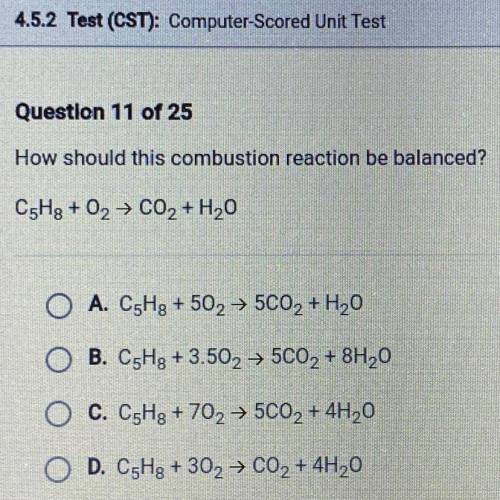
How should this combustion reaction be balanced?
C5Hg + 02 → C02 + H2O
A. C5Hg + 502 →...


Answers: 1
Another question on Chemistry

Chemistry, 20.06.2019 18:04
If this equation was completed which statement would it best support
Answers: 2

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1
You know the right answer?
Questions


Mathematics, 01.03.2021 18:40

Mathematics, 01.03.2021 18:40


Social Studies, 01.03.2021 18:40


Physics, 01.03.2021 18:40


Chemistry, 01.03.2021 18:40

Mathematics, 01.03.2021 18:40

Mathematics, 01.03.2021 18:40

Mathematics, 01.03.2021 18:40

English, 01.03.2021 18:40

Biology, 01.03.2021 18:40



Mathematics, 01.03.2021 18:40

Mathematics, 01.03.2021 18:40

English, 01.03.2021 18:40

Mathematics, 01.03.2021 18:40