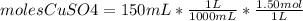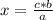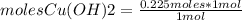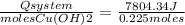Chemistry, 24.08.2019 12:30 kaitlngley2367
A150.0 ml sample of a 1.50 m solution of cuso4 is mixed with a 150.0 ml sample of 3.00 m koh in a coffee cup calorimeter. the temperature of both solutions and the calorimeter was
25.2°c before mixing and 31.3°c after mixing. the heat capacity of the calorimeter is 24.2 j/k.
calculate the δhrxn for this reaction in units of kj / mol of copper (ii) hydroxide (19 points). assume
the solutions is dilute enough that the specific heat and density of the solution is the same as that
of water (

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
A150.0 ml sample of a 1.50 m solution of cuso4 is mixed with a 150.0 ml sample of 3.00 m koh in a co...
Questions


Mathematics, 25.08.2019 14:10




Mathematics, 25.08.2019 14:10

History, 25.08.2019 14:10

Biology, 25.08.2019 14:10

English, 25.08.2019 14:10

History, 25.08.2019 14:10


History, 25.08.2019 14:10

Computers and Technology, 25.08.2019 14:10



Mathematics, 25.08.2019 14:10




Mathematics, 25.08.2019 14:10

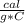 =4.184
=4.184 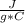 because the specific heat and density of the solution is the same as that
of water
Assuming that the total volume is the sum of the individual volumes then:
because the specific heat and density of the solution is the same as that
of water
Assuming that the total volume is the sum of the individual volumes then: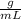 ) then
) then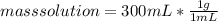
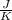 * [(31.3+273.15) - (25.2+273.15)] K = 147.62 J
* [(31.3+273.15) - (25.2+273.15)] K = 147.62 J