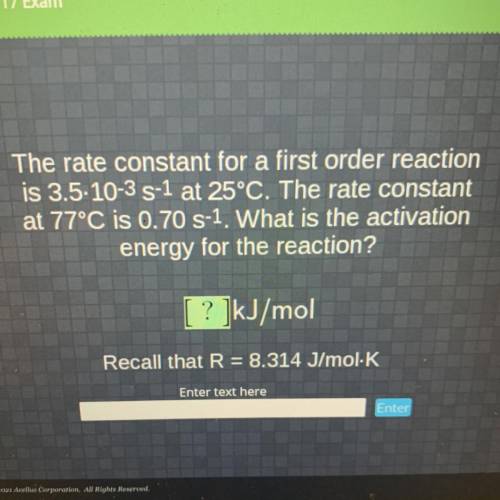
The rate constant for a first order reaction
is 3.5-10-3 S-1 at 25°C. The rate constant
at 77...

Chemistry, 03.03.2021 22:00 ryanzl1291
The rate constant for a first order reaction
is 3.5-10-3 S-1 at 25°C. The rate constant
at 77°C is 0.70 S-1. What is the activation
energy for the reaction?
[ ? ]kJ/mol
Recall that R = 8.314 J/mol K
-hurry it’s a test

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2


Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
Questions

Business, 02.02.2021 22:20

Mathematics, 02.02.2021 22:20

Advanced Placement (AP), 02.02.2021 22:20

History, 02.02.2021 22:20





Health, 02.02.2021 22:20

Mathematics, 02.02.2021 22:20

Mathematics, 02.02.2021 22:20


Mathematics, 02.02.2021 22:20



History, 02.02.2021 22:20


Social Studies, 02.02.2021 22:20

History, 02.02.2021 22:20

Mathematics, 02.02.2021 22:20



