
Chemistry, 04.03.2021 06:40 samueldfhung
Reactants - Products Reactants Products
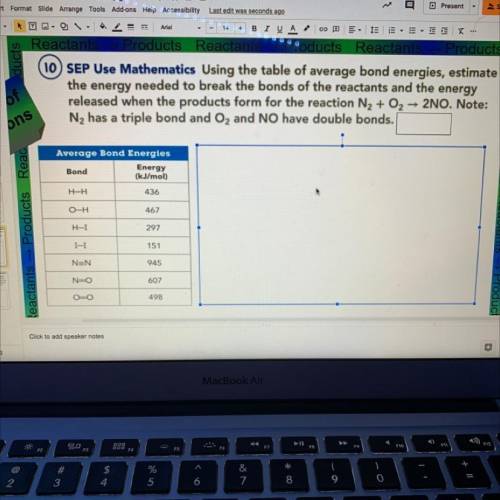
10 SEP Use Mathematics Using the table of average bond energies, estimate
the energy needed to break the bonds of the reactants and the energy
released when the products form for the reaction N2 + O2 2NO. Note:
N2 has a triple bond and O2 and NO have double bonds.
Reactants
of
ons
Average Bond Energies
Bond
Energy
(kJ/mol)
H-H
436
Products Reactant
O-H
467
H-I
297
I-I
151
eactants Products
NEN
945
N-O
607
→ Product
OO
498
Click to add speaker notes

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1
You know the right answer?
Reactants - Products Reactants Products
10 SEP Use Mathematics Using the table of average bond ener...
Questions

Arts, 27.01.2020 17:31



Computers and Technology, 27.01.2020 17:31

Mathematics, 27.01.2020 17:31


Computers and Technology, 27.01.2020 17:31

History, 27.01.2020 17:31




Mathematics, 27.01.2020 17:31

English, 27.01.2020 17:31



Mathematics, 27.01.2020 17:31



Mathematics, 27.01.2020 17:31



