
Chemistry, 04.03.2021 19:30 michael4443
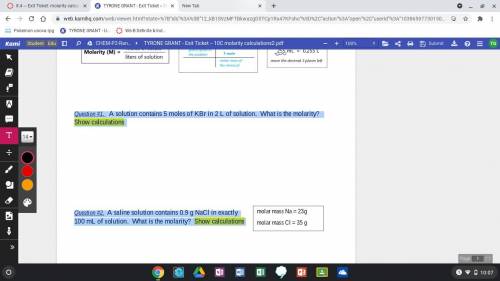
Question #1. A solution contains 5 moles of KBr in 2 L of solution. What is the molarity? Show calculations Question #2. A saline solution contains 0.9 g NaCl in exactly 100 mL of solution. What is the molarity? Show calculations

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
You know the right answer?
Question #1. A solution contains 5 moles of KBr in 2 L of solution. What is the molarity? Show calcu...
Questions


English, 09.10.2019 14:10

History, 09.10.2019 14:10


Mathematics, 09.10.2019 14:10

Physics, 09.10.2019 14:10

Mathematics, 09.10.2019 14:10


English, 09.10.2019 14:10



History, 09.10.2019 14:10


Chemistry, 09.10.2019 14:10

Biology, 09.10.2019 14:10


Biology, 09.10.2019 14:10


History, 09.10.2019 14:10

Computers and Technology, 09.10.2019 14:10



