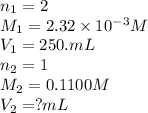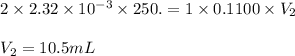Achemistry student weighs out 0.0475g of sulfurous acid h2so3, a diprotic acid, into a
250.ml...

Achemistry student weighs out 0.0475g of sulfurous acid h2so3, a diprotic acid, into a
250.ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1100m naoh solution.
calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
Questions





Mathematics, 24.04.2021 03:10


Mathematics, 24.04.2021 03:10



Chemistry, 24.04.2021 03:10

Mathematics, 24.04.2021 03:10


English, 24.04.2021 03:10

Chemistry, 24.04.2021 03:10

Mathematics, 24.04.2021 03:10

Mathematics, 24.04.2021 03:10

Mathematics, 24.04.2021 03:20

Mathematics, 24.04.2021 03:20

Mathematics, 24.04.2021 03:20

English, 24.04.2021 03:20

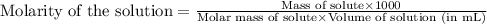
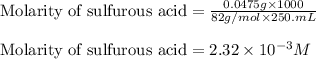
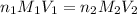
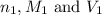 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 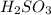
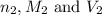 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.