Chemistry, 04.03.2021 20:00 jennaranelli05
Calcium carbonate decomposes to form calcium oxide and carbon dioxide, like this:
CaCO3(s)→CaO(s)+CO2(g)
At a certain temperature, a chemist finds that a 9.0L reaction vessel containing a mixture of calcium carbonate, calcium oxide, and carbon dioxide at equilibrium has the following composition:
Compound Amount
CaCO3 25.3 g
CaO 14.9 g
CO2 33.7 g
Calculate the value of the equilibrium constant Kc for this reaction. Round your answer to 2 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1


Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1
You know the right answer?
Calcium carbonate decomposes to form calcium oxide and carbon dioxide, like this:
CaCO3(s)→CaO(s)+C...
Questions

Physics, 08.05.2021 15:00

Mathematics, 08.05.2021 15:00


Physics, 08.05.2021 15:00

Mathematics, 08.05.2021 15:00


Mathematics, 08.05.2021 15:00


Mathematics, 08.05.2021 15:00


History, 08.05.2021 15:10


Mathematics, 08.05.2021 15:10


Mathematics, 08.05.2021 15:10

Mathematics, 08.05.2021 15:10




Mathematics, 08.05.2021 15:10

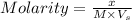
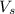 = volume of solution in L
= volume of solution in L
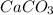 =
= 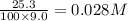
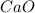 =
= 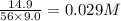
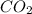 =
= 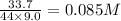
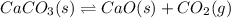
![K_c=\frac{[CaO]\times [CO_2]}{[CaCO_3]}](/tpl/images/1168/6891/ed17d.png)