
Chemistry, 04.03.2021 21:00 brooket30057
2. You can calculate the solubility of a metal carbonate in pure water using the Ksp
of the sparingly soluble salt and the Kb expression for the hydrolysis of carbonate
ion.
This leads to the pseudo-quadratic equation in which x is the solubility of metal
carbonate.
*? - VK, K,- K,, -
= 0
Consider CaCO3. Where the Ksp=4.7x10-9 with the carbonate Kb = 2.1x10-4
Use the method of successive approximations to the find the solubility of CaCO3
to at least three significant figures.
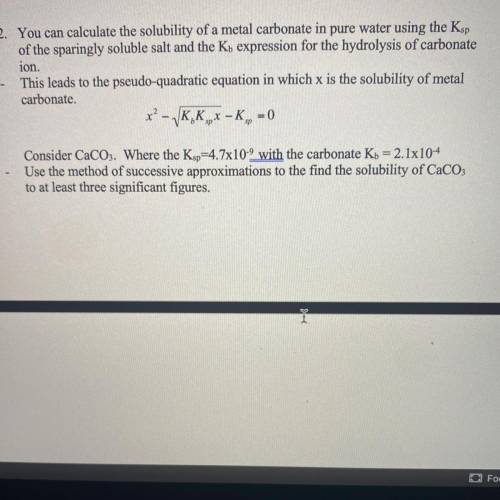

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
2. You can calculate the solubility of a metal carbonate in pure water using the Ksp
of the sparing...
Questions


English, 09.08.2021 23:20











Computers and Technology, 09.08.2021 23:20









