
Chemistry, 04.03.2021 21:40 kerstynsharp08
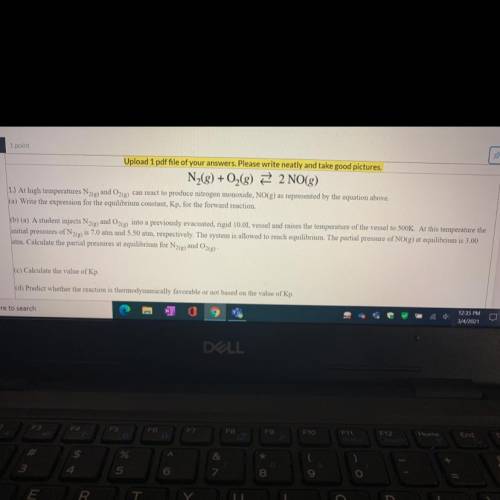
N2 + O2 —> 2NO
b) A student injects N2(g)and O2(g) into a previously evacuated, rigid 10.0L vessel and raises the temperature of the vessel to 500K. At this temperature the
initial pressures of N2(g) is 7.0 atm and 5.50 atm, respectively. The system is allowed to reach equilibrium. The partial pressure of NO(g) at equilibrium is 3.00
atm. Calculate the partial pressures at equilibrium for N2(e) and O2(g)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
N2 + O2 —> 2NO
b) A student injects N2(g)and O2(g) into a previously evacuated, rigid 10.0L vess...
Questions





Mathematics, 15.07.2020 14:01

English, 15.07.2020 14:01


Mathematics, 15.07.2020 14:01

History, 15.07.2020 14:01

English, 15.07.2020 14:01

History, 15.07.2020 14:01

Biology, 15.07.2020 14:01


Mathematics, 15.07.2020 14:01

Mathematics, 15.07.2020 14:01



Mathematics, 15.07.2020 14:01




