When limestone (which is principally CaCO3)
is heated, carbon dioxide and quicklime
(Cao) are...

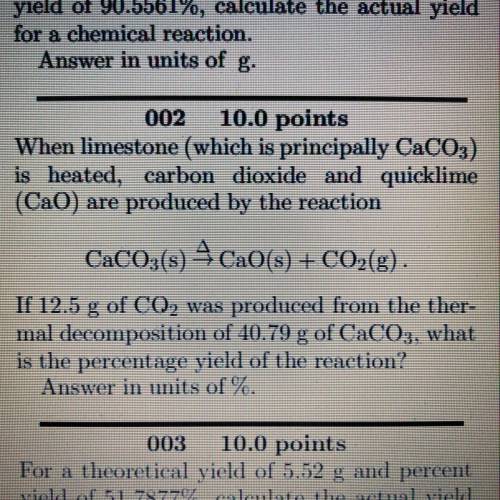
When limestone (which is principally CaCO3)
is heated, carbon dioxide and quicklime
(Cao) are produced by the reaction
CaCO3(s) 4CaO(s) + CO2(g).
If 12.5 g of CO2 was produced from the ther-
mal decomposition of 40.79 g of CaCO3, what
is the percentage yield of the reaction?
Answer in units of %.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

You know the right answer?
Questions

Mathematics, 21.08.2019 13:30


Mathematics, 21.08.2019 13:30

French, 21.08.2019 13:30

Geography, 21.08.2019 13:30





Mathematics, 21.08.2019 13:30


Mathematics, 21.08.2019 13:30


Computers and Technology, 21.08.2019 13:30

History, 21.08.2019 13:30

Mathematics, 21.08.2019 13:30


Mathematics, 21.08.2019 13:30




