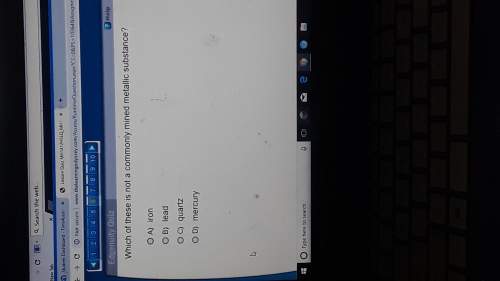
Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
A 170.00 g sample of an unidentified compound contains 29.84 g sodium, 67.49 g chromium, and
72.67...
Questions






Physics, 20.09.2021 05:20



Biology, 20.09.2021 05:20

Social Studies, 20.09.2021 05:20

English, 20.09.2021 05:20





Mathematics, 20.09.2021 05:20

English, 20.09.2021 05:20

Mathematics, 20.09.2021 05:20