
Chemistry, 05.03.2021 02:40 chazpooh208
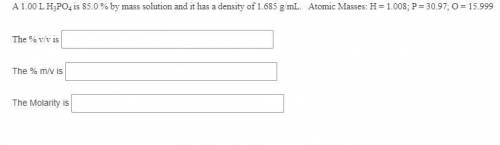
A 1.00 L H3PO4 is 85.0 % by mass solution and it has a density of 1.685 g/mL. Atomic Masses: H = 1.008; P = 30.97; O = 15.999

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
You know the right answer?
A 1.00 L H3PO4 is 85.0 % by mass solution and it has a density of 1.685 g/mL. Atomic Masses: H = 1.0...
Questions



Mathematics, 03.12.2020 20:30

Mathematics, 03.12.2020 20:30

Mathematics, 03.12.2020 20:30

English, 03.12.2020 20:30



Computers and Technology, 03.12.2020 20:30


Geography, 03.12.2020 20:30


History, 03.12.2020 20:30


Mathematics, 03.12.2020 20:30


Mathematics, 03.12.2020 20:30


English, 03.12.2020 20:30



