
Chemistry, 05.03.2021 04:00 ariloveshorses
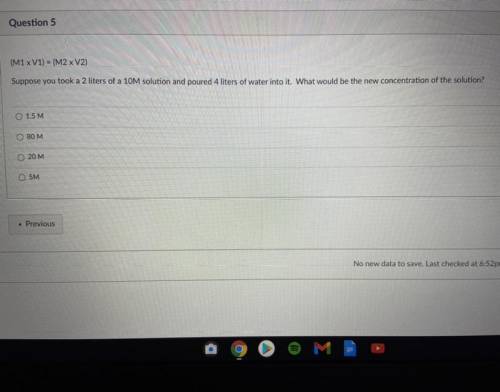
Suppose you a 2 of 10M solution and poured 4 liters of water into it. What would be the new concentration of the solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1
You know the right answer?
Suppose you a 2 of 10M solution and poured 4 liters of water into it. What would be the new concentr...
Questions

Mathematics, 02.02.2021 22:40

Mathematics, 02.02.2021 22:40

History, 02.02.2021 22:40



Chemistry, 02.02.2021 22:40

English, 02.02.2021 22:40

Mathematics, 02.02.2021 22:40


Social Studies, 02.02.2021 22:40

Mathematics, 02.02.2021 22:40

Social Studies, 02.02.2021 22:40




Biology, 02.02.2021 22:40


Social Studies, 02.02.2021 22:40


Mathematics, 02.02.2021 22:40



