
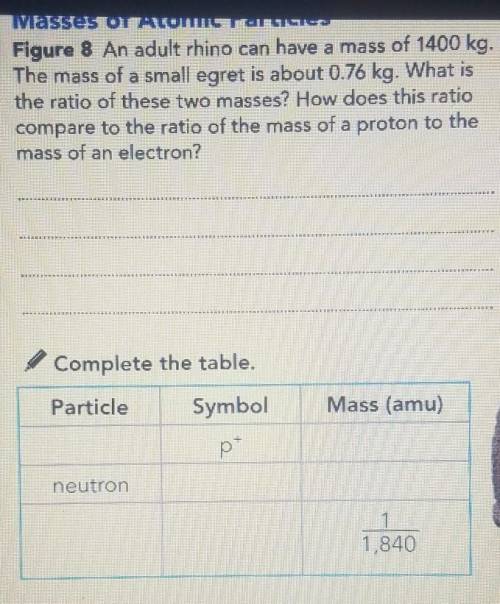
Masses of Atomic Particles Figure 8 An adult rhino can have a mass of 1400 kg. The mass of a small egret is about 0.76 kg. What is the ratio of these two masses? How does this ratio compare to the ratio of the mass of a proton to the mass of an electron? MIKRO SKIRTS ki KAMI Complete the table. Particle Symbol Mass (amu) p neutron 1,840 1 1,840

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3


Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
Masses of Atomic Particles Figure 8 An adult rhino can have a mass of 1400 kg. The mass of a small e...
Questions

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

English, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

History, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01

Mathematics, 10.09.2020 14:01



