CH2(g) + 2Cl2(g) → CCl4(g) + 2H2(g)
AH T, CH, = -74.8 kJ/mol
AH 1. CCI, = -106.7 kJ/mol
...

Chemistry, 05.03.2021 07:00 HaydenSturgis1
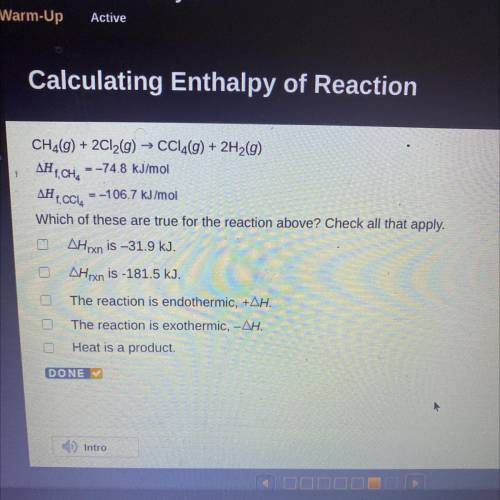
CH2(g) + 2Cl2(g) → CCl4(g) + 2H2(g)
AH T, CH, = -74.8 kJ/mol
AH 1. CCI, = -106.7 kJ/mol
Which of these are true for the reaction above? Check all that apply.
AHrxn is –31.9 kJ.
AHrxn is -181.5 kJ.
The reaction is endothermic, +AH.
The reaction is exothermic, -AH.
Heat is a product.
PLEASE HELP IM BEGGING AND I WILL GIVE BRAINLIEST

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3

Chemistry, 23.06.2019 14:00
Which of the following represents the balanced reduction half-reaction from the redox reaction? (2 points) pb + pd(no3)2 yields pb(no3)2 + pd pb yields pd2+ + e- pd2+ + 2e- yields pd pb + e- yields pb 2 pd2+ + 4 e- yields 2 pd
Answers: 1


Chemistry, 23.06.2019 19:00
What is the final temperature after 840 joules is absorbed by 10.0g of water at 25.0c
Answers: 1
You know the right answer?
Questions




Mathematics, 11.01.2021 17:00



Mathematics, 11.01.2021 17:00

History, 11.01.2021 17:00

Health, 11.01.2021 17:00

Physics, 11.01.2021 17:00

Mathematics, 11.01.2021 17:00

Mathematics, 11.01.2021 17:00


History, 11.01.2021 17:00


English, 11.01.2021 17:00

Biology, 11.01.2021 17:00



Mathematics, 11.01.2021 17:00



