
Chemistry, 05.03.2021 06:50 rleiphart1
When 1.50 mol CO2 and 1.50 mol H2 are placed in a 3.00-Lcontainer at 395 °C, the following reaction
CO2(g) + H2(g) = CO(g) + H2O(g). If Kc = 0.802,
what are the concentrations of each substance in the equilibrium mixture?
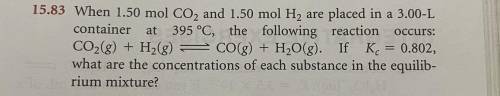

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
When 1.50 mol CO2 and 1.50 mol H2 are placed in a 3.00-Lcontainer at 395 °C, the following reaction...
Questions

Mathematics, 28.05.2020 07:57


Mathematics, 28.05.2020 07:57



Mathematics, 28.05.2020 07:57

Mathematics, 28.05.2020 07:57










Mathematics, 28.05.2020 07:57



Mathematics, 28.05.2020 07:57



