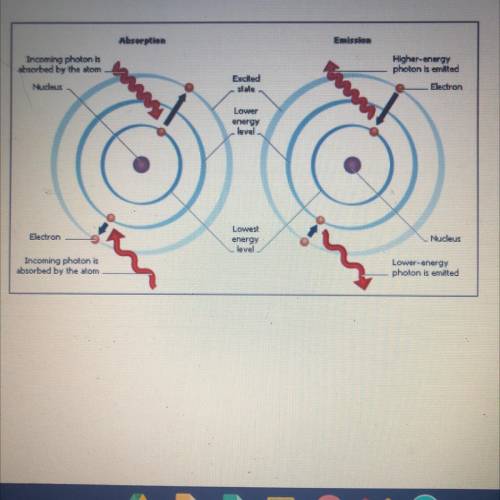
Q.1 When a metal is heated, what
happens to the electrons in
the atoms?
Q.2 What is th...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
Questions


Social Studies, 13.01.2021 23:20

English, 13.01.2021 23:20

Health, 13.01.2021 23:20

English, 13.01.2021 23:20


Mathematics, 13.01.2021 23:20

Arts, 13.01.2021 23:20

Chemistry, 13.01.2021 23:20


History, 13.01.2021 23:20

Mathematics, 13.01.2021 23:20


Physics, 13.01.2021 23:20


Mathematics, 13.01.2021 23:20

English, 13.01.2021 23:20

Social Studies, 13.01.2021 23:20


Mathematics, 13.01.2021 23:20