
Chemistry, 05.03.2021 19:30 dogsrdabest
At 5.0 C the value of Kw for the equilibrium shown above is 1.9 X 10^-15 and the value of pKw is 14.73 . Based on this information , which of the following is correct for pure water at this temperature?
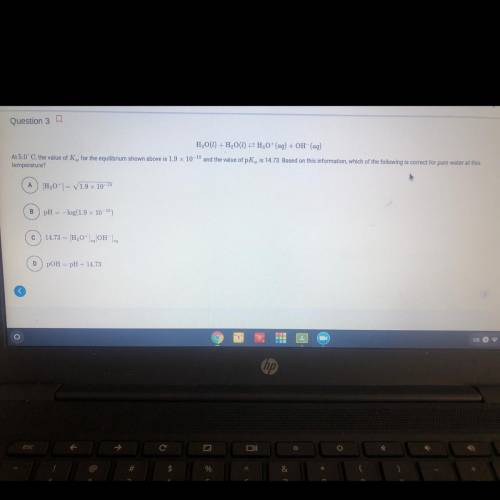

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
At 5.0 C the value of Kw for the equilibrium shown above is 1.9 X 10^-15 and the value of pKw is 14....
Questions


History, 16.10.2020 18:01


Spanish, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01

Computers and Technology, 16.10.2020 18:01

Health, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01

Biology, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01




History, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01



