Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
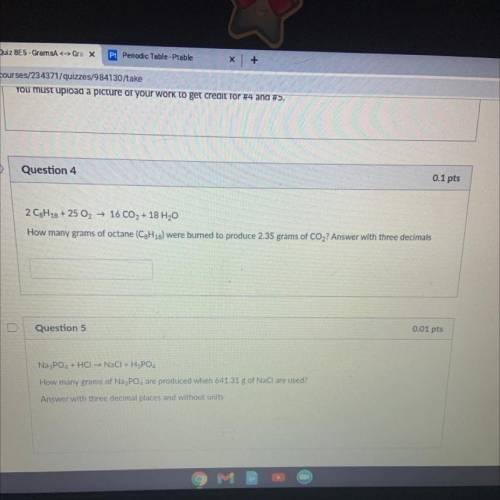
2 C8H18 + 25 O2 → 16 CO2 + 18 H20
How many grams of octane (C3H18) were burned to produce 2.35 gram...
Questions


Business, 13.03.2021 14:00

Biology, 13.03.2021 14:00


Mathematics, 13.03.2021 14:00


English, 13.03.2021 14:00

Mathematics, 13.03.2021 14:00

Mathematics, 13.03.2021 14:00

Mathematics, 13.03.2021 14:00

Physics, 13.03.2021 14:00


Health, 13.03.2021 14:00

Mathematics, 13.03.2021 14:00

Mathematics, 13.03.2021 14:00

Engineering, 13.03.2021 14:00

Spanish, 13.03.2021 14:00


Computers and Technology, 13.03.2021 14:00

Mathematics, 13.03.2021 14:00