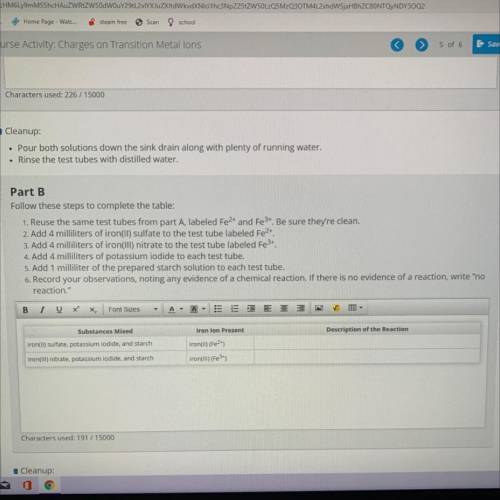
Part B
Follow these steps to complete the table:
1. Reuse the same test tubes from part A, la...

Chemistry, 05.03.2021 22:30 xxQueenPxx7432
Part B
Follow these steps to complete the table:
1. Reuse the same test tubes from part A, labeled Fe2+ and Fe3+. Be sure they're clean.
2. Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2+.
3. Add 4 milliliters of iron(III) nitrate to the test tube labeled Fe3+.
4. Add 4 milliliters of potassium iodide to each test tube.
5. Add 1 milliliter of the prepared starch solution to each test tube.
6. Record your observations, noting any evidence of a chemical reaction. If there is no evidence of a reaction, w
reaction."
B 1
U
x
x х.
Font Sizes
AA. EE
Substances Mixed
Iron lon Present
Description of the Reaction
iron(II) sulfate, potassium iodide, and starch
iron(ul) (Fe2+)
iron(III) (Fe 3+)
iron(III) nitrate, potassium iodide, and starch

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1


Chemistry, 23.06.2019 06:30
When microscope slides are stained to show blood cells, the small red blood cells that appear on the slides are much numerous than the large white blood cells. this supports the concept that
Answers: 1

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
You know the right answer?
Questions


Health, 05.10.2019 10:50


History, 05.10.2019 10:50


English, 05.10.2019 10:50

Biology, 05.10.2019 10:50

Chemistry, 05.10.2019 10:50

Biology, 05.10.2019 10:50



English, 05.10.2019 11:00




Biology, 05.10.2019 11:00

Chemistry, 05.10.2019 11:00





