Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
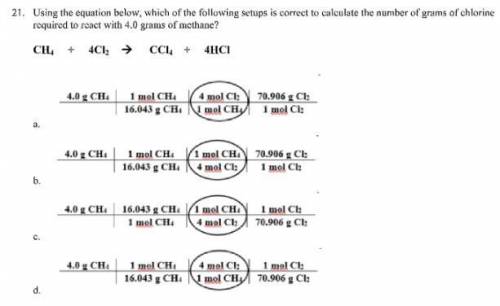
Using the equation below, which of the following setups is correct to calculate the number of grams...
Questions


Spanish, 23.09.2019 02:20

Mathematics, 23.09.2019 02:20

Computers and Technology, 23.09.2019 02:20


Mathematics, 23.09.2019 02:20

Mathematics, 23.09.2019 02:20

History, 23.09.2019 02:20



Mathematics, 23.09.2019 02:20


Biology, 23.09.2019 02:20


Chemistry, 23.09.2019 02:20


History, 23.09.2019 02:20

Mathematics, 23.09.2019 02:20

Biology, 23.09.2019 02:20