
Chemistry, 08.03.2021 14:00 24hudsonmoss
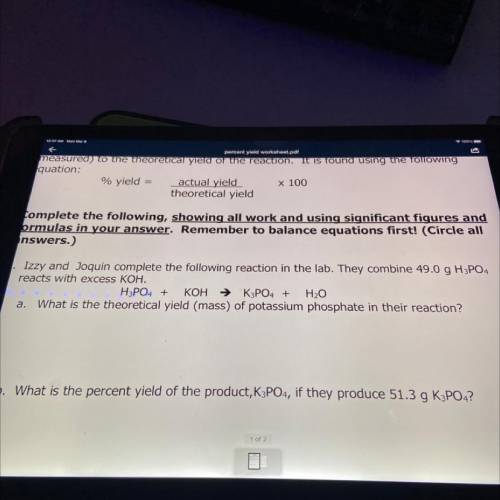
Izzy and Joquin complete the following reaction in the lab. They combine 49.0 g H3PO4
reacts with excess KOH.
H3PO4 + KOH → K3PO4 + H2O
a. What is the theoretical yield (mass) of potassium phosphate in their reaction?
b. What is the percent yield of the product, K3PO4, if they produce 51.3 g K3PO4?
1 of 2

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3

Chemistry, 23.06.2019 07:00
Ajar contains a certain substance. which observation would show that the substance must be either a solid or a liquid?
Answers: 1

Chemistry, 23.06.2019 09:00
What factor besides temperature affects the boiling point of water? a. mass b. number of moles c. volume d. pressure
Answers: 3
You know the right answer?
Izzy and Joquin complete the following reaction in the lab. They combine 49.0 g H3PO4
reacts with e...
Questions


Mathematics, 28.01.2020 13:33

History, 28.01.2020 13:33

History, 28.01.2020 13:33

History, 28.01.2020 13:33





Mathematics, 28.01.2020 13:33

Health, 28.01.2020 13:33



Mathematics, 28.01.2020 13:33

Biology, 28.01.2020 13:33

History, 28.01.2020 13:33


History, 28.01.2020 13:33




