
Chemistry, 08.03.2021 18:10 kaylarose7658
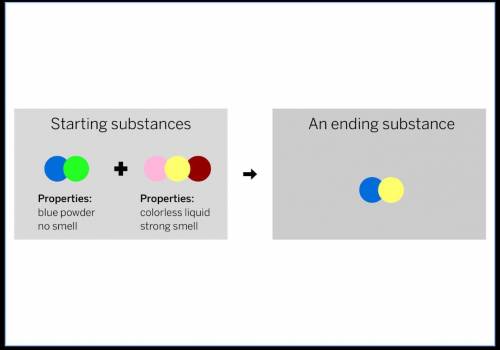
A chemist mixed two substances together: a blue powder with no smell and a colorless liquid with a strong smell. Their repeating groups of atoms are shown above on the left. After they were mixed, the chemist analyzed the results and found two substances. One ending substance had the repeating group of atoms shown above on the right. Is the ending substance the same substance as the blue powder? What happened to the atoms of the starting substances when the ending substances formed? Be sure to explain your answers to both of these questions.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
A chemist mixed two substances together: a blue powder with no smell and a colorless liquid with a s...
Questions


History, 10.08.2021 16:40



Mathematics, 10.08.2021 16:40


English, 10.08.2021 16:40




English, 10.08.2021 16:50

Chemistry, 10.08.2021 16:50



Mathematics, 10.08.2021 16:50

English, 10.08.2021 16:50


History, 10.08.2021 16:50

English, 10.08.2021 16:50

English, 10.08.2021 16:50



