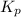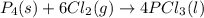Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 07:30
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3

Chemistry, 23.06.2019 15:00
What do we call the rows on the periodic table? a. periodb. familyc. groupd. metals
Answers: 1
You know the right answer?
Equilibrium constant Kp for the reaction P4(s) + 6Cl2(g) 4PCl3(l) Kp = 1/ P6Cl2 Kp = P4Cl3/ P6P4 Kp...
Questions

Mathematics, 04.02.2021 19:40



Mathematics, 04.02.2021 19:40

SAT, 04.02.2021 19:40


History, 04.02.2021 19:40

Mathematics, 04.02.2021 19:40

Mathematics, 04.02.2021 19:40


Mathematics, 04.02.2021 19:40




Mathematics, 04.02.2021 19:40


Mathematics, 04.02.2021 19:40



![K_p=\frac{1}{[p_{Cl_2}]^6}](/tpl/images/1177/2723/ab503.png)