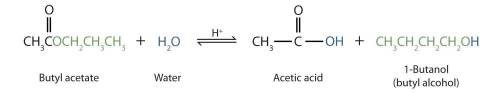
Esters may undergo a process called hydrolysis in which an ester is split by water. Hydrolysis of esters can occur in the presence of strong acids or strong bases. When heated in the presence of a strong acid such as H2SO4, water splits an ester into a carboxylic acid and an alcohol. This process is called acid hydrolysis. In the presence of a strong base such as NaOH, water splits an ester into a carboxylic acid salt and an alcohol. This process is called base hydrolysis or saponification.
Required:
Draw the carboxylic acid produced from the acid hydrolysis of butyl acetate.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

You know the right answer?
Esters may undergo a process called hydrolysis in which an ester is split by water. Hydrolysis of es...
Questions

Physics, 24.09.2020 05:01

Mathematics, 24.09.2020 05:01

English, 24.09.2020 05:01

Mathematics, 24.09.2020 05:01


Mathematics, 24.09.2020 05:01

Mathematics, 24.09.2020 05:01




Mathematics, 24.09.2020 05:01


History, 24.09.2020 05:01


Mathematics, 24.09.2020 05:01


Engineering, 24.09.2020 05:01

Computers and Technology, 24.09.2020 05:01


Computers and Technology, 24.09.2020 05:01