
Chemistry, 08.03.2021 22:00 anitadefrances
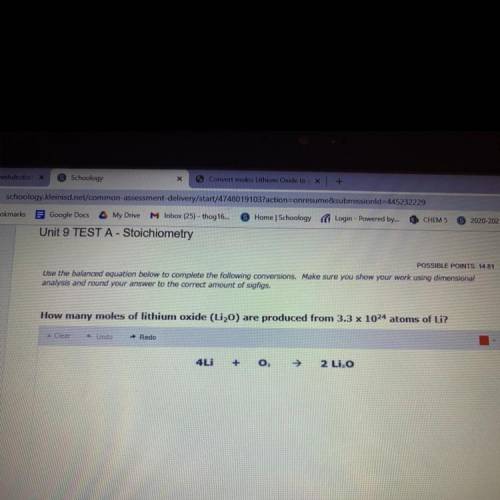
POSSIBLE POINTS: 14.81
Use the balanced equation below to complete the following conversions. Make sure you show your work using dimensional
analysis and round your answer to the correct amount of sigfigs.
How many moles of lithium oxide (Li2O) are produced from 3.3 x 1024 atoms of Li?
Helpp?!? ASAP Work shown

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2
You know the right answer?
POSSIBLE POINTS: 14.81
Use the balanced equation below to complete the following conversions. Make...
Questions

Computers and Technology, 02.12.2020 01:00



Mathematics, 02.12.2020 01:00


Arts, 02.12.2020 01:00



English, 02.12.2020 01:00

Mathematics, 02.12.2020 01:00

Social Studies, 02.12.2020 01:00

Mathematics, 02.12.2020 01:00


Mathematics, 02.12.2020 01:00


English, 02.12.2020 01:00

Mathematics, 02.12.2020 01:00



Spanish, 02.12.2020 01:00



