
Chemistry, 08.03.2021 22:00 allisonklinger1786
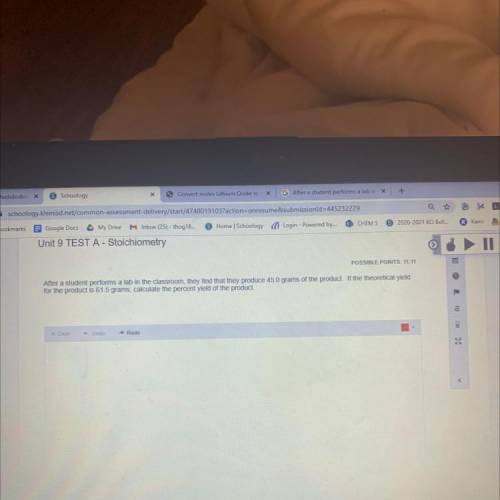
After a student performs a lab in the classroom, they find that they produce 45.0 grams of the product. If the theoretical yield
for the product is 61.5 grams, calculate the percent yield of the product
HELP ASAP

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3


Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3

Chemistry, 23.06.2019 14:00
Which is not true regarding reaction rates? (2 points) catalysts are not used up in the reaction. catalysts speed up reactions by lowering the activation energy. reaction rates decrease as the concentration of reactants decrease. during reactions, concentrations of all reactants decrease at the same rate.
Answers: 1
You know the right answer?
After a student performs a lab in the classroom, they find that they produce 45.0 grams of the produ...
Questions



Social Studies, 08.10.2019 18:10







English, 08.10.2019 18:10







History, 08.10.2019 18:10





