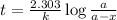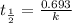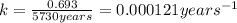Chemistry, 08.03.2021 23:10 robert7248
The previous part could be done without using the decay equation, because the ratio of original 14C14C to present 14C14C was an integer power of 1/2. Most problems are not so simple. To solve more general carbon-dating problems, you must first find the value of the decay constant for 14C14C, so that you can easily use the decay equation. Using the given half-life, 5730 yearsyears, find the value of the decay constant for 14C14C. Express your answer in inverse years to three significant figures. View Available Hint(s)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

You know the right answer?
The previous part could be done without using the decay equation, because the ratio of original 14C1...
Questions

Mathematics, 26.12.2019 18:31

Health, 26.12.2019 18:31

Mathematics, 26.12.2019 18:31

Health, 26.12.2019 18:31

Mathematics, 26.12.2019 18:31

Mathematics, 26.12.2019 18:31











Computers and Technology, 26.12.2019 18:31



Computers and Technology, 26.12.2019 18:31