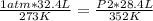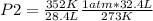Chemistry, 09.03.2021 02:50 lilbopeep21
A 32.4 L gas sample at STP is compressed to a volume of 28.4 L, and the temperature is increased to 352 K.
What is the new pressure of the gas in atmospheres? 1.47atm
How do I solve this?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

You know the right answer?
A 32.4 L gas sample at STP is compressed to a volume of 28.4 L, and the temperature is increased to...
Questions

History, 08.09.2021 07:20

Computers and Technology, 08.09.2021 07:20

Social Studies, 08.09.2021 07:20


Mathematics, 08.09.2021 07:30

Social Studies, 08.09.2021 07:30



History, 08.09.2021 07:30

Mathematics, 08.09.2021 07:30

Mathematics, 08.09.2021 07:30

Mathematics, 08.09.2021 07:30





Mathematics, 08.09.2021 07:30