
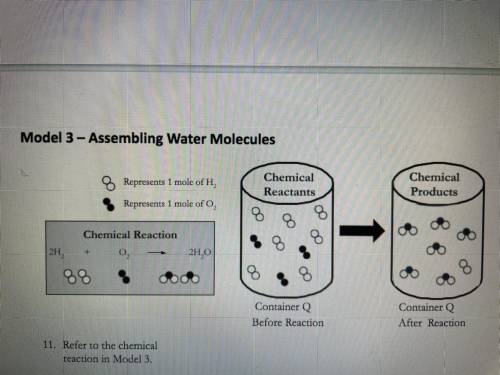
Consider the synthesis of water as shown in Model 3. A container is filled with 10.0 g of H, and
5.0 g of O,
a. Which reactant (hydrogen or oxygen) is the limiting reactant in this case? Show your work.
Hint: Notice that you are given reactant quantities in mass units here, not moles.
b. What mass of water can be produced? Show your work.
c. Which reactant is present in excess, and what mass of that reactant remains after the
reaction is complete? Show your work.
I

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
Consider the synthesis of water as shown in Model 3. A container is filled with 10.0 g of H, and
5....
Questions

Mathematics, 25.03.2021 18:10


Chemistry, 25.03.2021 18:10

Mathematics, 25.03.2021 18:10



Mathematics, 25.03.2021 18:10



English, 25.03.2021 18:10




Mathematics, 25.03.2021 18:10



Mathematics, 25.03.2021 18:10


Mathematics, 25.03.2021 18:10

Biology, 25.03.2021 18:10



