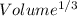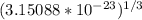Tungsten and molybdenum both have the BCC crystal structure, and Mo forms a substitutional solid solution for all concentrations at room temperature. Compute the unit cell edge length for a 84 wt% W - 16 wt% Mo alloy. The room-temperature density and atomic weight of W are 19.3 g/cm3 and 183.85 g/mol, the room-temperature density and atomic weight of Mo are 10.22 g/cm3 and 95.94 g/mol, respectively.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
Tungsten and molybdenum both have the BCC crystal structure, and Mo forms a substitutional solid sol...
Questions



Law, 12.09.2019 00:30

English, 12.09.2019 00:30





Chemistry, 12.09.2019 00:30

Mathematics, 12.09.2019 00:30


Mathematics, 12.09.2019 00:30



Mathematics, 12.09.2019 00:30

History, 12.09.2019 00:30

Mathematics, 12.09.2019 00:30

Mathematics, 12.09.2019 00:30

Spanish, 12.09.2019 00:30

Social Studies, 12.09.2019 00:30