
Chemistry, 09.03.2021 05:50 NutMeg6544
The equilibrium constant, Kc, is calculated using molar concentrations. For gaseous reactions another form of the equilibrium constant, Kp, is calculated from partial pressures instead of concentrations. These two equilibrium constants are related by the equation Kp=Kc(RT)Δn where R=0.08206 L⋅atm/(K⋅mol), T is the absolute temperature, and Δn is the change in the number of moles of gas (sum moles products - sum moles reactants). For example, consider the reaction N2(g)+3H2(g)⇌2NH3(g) for which Δn=2−(1+3)=−2. Part A For the reaction 2A(g)+3B(g)⇌C(g) Kc = 63.2 at a temperature of 81 ∘C . Calculate the value of Kp. Express your answer numerically.

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3

Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1

Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3
You know the right answer?
The equilibrium constant, Kc, is calculated using molar concentrations. For gaseous reactions anothe...
Questions

Mathematics, 26.02.2021 14:00

French, 26.02.2021 14:00

Mathematics, 26.02.2021 14:00

Mathematics, 26.02.2021 14:00

Mathematics, 26.02.2021 14:00

Mathematics, 26.02.2021 14:00


Mathematics, 26.02.2021 14:00

English, 26.02.2021 14:00




Mathematics, 26.02.2021 14:00


History, 26.02.2021 14:00



Chemistry, 26.02.2021 14:00


Social Studies, 26.02.2021 14:00

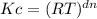 = (0.082 L.atm/K.mol x 354 K)⁻⁴ = 1.41 x 10⁻⁶
= (0.082 L.atm/K.mol x 354 K)⁻⁴ = 1.41 x 10⁻⁶

