
One essential step in the manufacture of many plant fertilizers is the production of nitrogen monoxide gas, NO(g). This step is represented by the following balanced equation: 4 NH3(g) + 5 O2(g) --> 4 NO(g) + 6 H2O(l) Determine the amount of oxygen needed to produce 1.2 x 104 mol of nitrogen monoxide gas.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
One essential step in the manufacture of many plant fertilizers is the production of nitrogen monoxi...
Questions


History, 27.12.2020 05:40



Mathematics, 27.12.2020 05:40

History, 27.12.2020 05:40







Computers and Technology, 27.12.2020 05:50

Mathematics, 27.12.2020 05:50





Computers and Technology, 27.12.2020 05:50

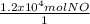 ×
× 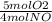 = 1.5 moles of O2 needed
= 1.5 moles of O2 needed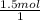 ×
× 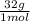 = 48 grams needed
= 48 grams needed

