
Chemistry, 09.03.2021 08:50 ashleyroberson735
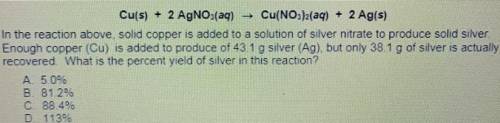
Cu(s) + 2 AgNO3(aq) Cu(NO3)2(aq) + 2 Ag(s)
14. In the reaction above solid copper is added to a solution of silver nitrate to produce solid silver.
Enough copper (Cu) is added to produce of 431 g silver (Ag) but only 38.1 g of silver is actually
recovered What is the percent yield of silver in this reaction?
A. 5.0%
B. 81.2%
C 88 46
D. 1139

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2


Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
Cu(s) + 2 AgNO3(aq) Cu(NO3)2(aq) + 2 Ag(s)
14. In the reaction above solid copper is added to a sol...
Questions

Mathematics, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50


Mathematics, 20.11.2020 19:50

Biology, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50




Law, 20.11.2020 19:50





Mathematics, 20.11.2020 19:50

Biology, 20.11.2020 19:50

History, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50

World Languages, 20.11.2020 19:50



