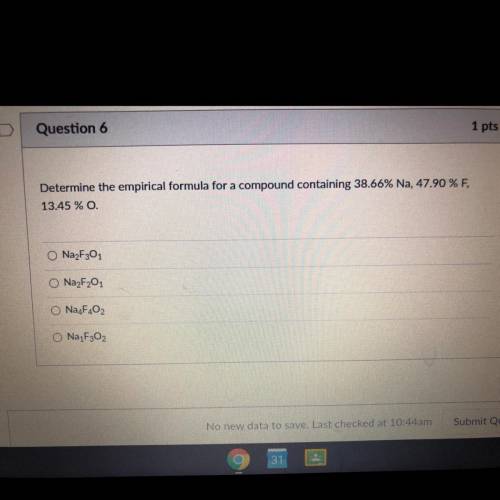
Determine the empirical formula for a compound containing 38.66% Na, 47.90 % F, 13.45 % 0.
...

Chemistry, 09.03.2021 22:20 montgomerykarloxc24x
Determine the empirical formula for a compound containing 38.66% Na, 47.90 % F, 13.45 % 0.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1

Chemistry, 23.06.2019 10:00
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1
You know the right answer?
Questions


Geography, 20.08.2021 17:40

Computers and Technology, 20.08.2021 17:40

Mathematics, 20.08.2021 17:40

Physics, 20.08.2021 17:40

Computers and Technology, 20.08.2021 17:40






Mathematics, 20.08.2021 17:40







Mathematics, 20.08.2021 17:40

Mathematics, 20.08.2021 17:40



