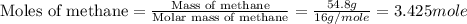Chemistry, 13.10.2019 05:01 averyeverdeen01
The molar heat of vaporization for methane, ch4, is 8.53 kj/mol. how much energy is absorbed when 54.8 g of methane vaporizes at its boiling point?
6.42 kj
29.1 kj
137 kj
467 kj

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2


Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3
You know the right answer?
The molar heat of vaporization for methane, ch4, is 8.53 kj/mol. how much energy is absorbed when 54...
Questions

Mathematics, 11.09.2020 18:01

Social Studies, 11.09.2020 18:01

Mathematics, 11.09.2020 18:01

Mathematics, 11.09.2020 18:01

Biology, 11.09.2020 18:01

Mathematics, 11.09.2020 18:01

Mathematics, 11.09.2020 18:01

Mathematics, 11.09.2020 18:01

Mathematics, 11.09.2020 18:01

Health, 11.09.2020 18:01

Geography, 11.09.2020 18:01

Mathematics, 11.09.2020 18:01

Mathematics, 11.09.2020 18:01

Mathematics, 11.09.2020 18:01

Mathematics, 11.09.2020 18:01

Spanish, 11.09.2020 18:01

Mathematics, 11.09.2020 18:01

Mathematics, 11.09.2020 18:01

Biology, 11.09.2020 19:01

English, 11.09.2020 19:01