
Chemistry, 10.03.2021 04:00 vrentadrienneoqug1a
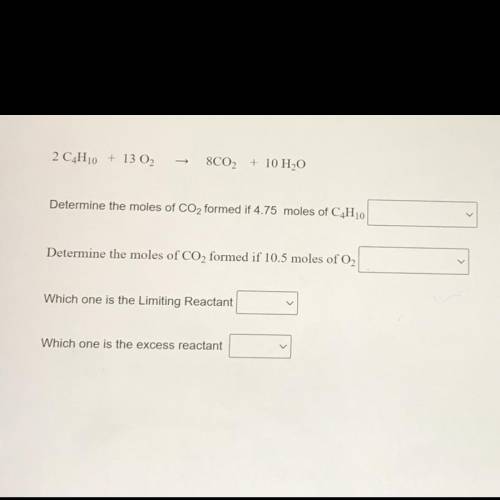
2 C4H10 + 13 02
8C02 + 10 H2O
Determine the moles of CO2 formed if 4.75 moles of C4H10
<
Determine the moles of CO2 formed if 10.5 moles of O2
Which one is the Limiting Reactant
Which one is the excess reactant

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Wind is related tot he movement or warm cold air masses which kind of heat transfer does this represent
Answers: 2

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1
You know the right answer?
2 C4H10 + 13 02
8C02 + 10 H2O
Determine the moles of CO2 formed if 4.75 moles of C4H10
...
Determine the moles of CO2 formed if 4.75 moles of C4H10
...
Questions


English, 02.07.2019 18:10


Mathematics, 02.07.2019 18:10



Mathematics, 02.07.2019 18:10


English, 02.07.2019 18:10

History, 02.07.2019 18:10

History, 02.07.2019 18:10


Mathematics, 02.07.2019 18:10

Mathematics, 02.07.2019 18:10


Geography, 02.07.2019 18:10







