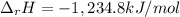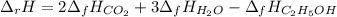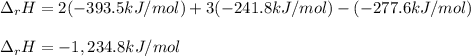Alcohol is used as a source of fuel for some automobiles. The standard molar enthalpy of formation of ethanol C2H5OH(l) is -277.6 kJ/mol. For the balanced reaction equation of ethanol use:
C2H5OH(l) + 3O2 --> 2CO2 + 3H2O.
Ethanol is a liquid, but all the other chemicals in this reaction are gases. What is the enthalpy change of this reaction in kJ/mol?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3
You know the right answer?
Alcohol is used as a source of fuel for some automobiles. The standard molar enthalpy of formation o...
Questions



Mathematics, 29.09.2020 14:01



Mathematics, 29.09.2020 14:01


English, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01




Mathematics, 29.09.2020 14:01

Health, 29.09.2020 14:01