
Chemistry, 11.03.2021 14:10 kaelynnmarie1135
Calcium chloride can be prepared by the reaction of calcium with chlorine gas.
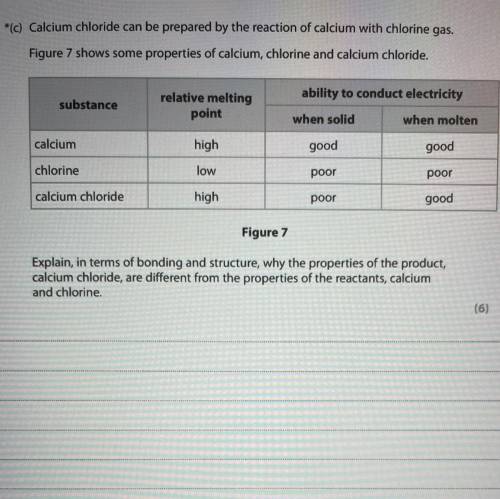
Figure 7 shows some properties of calcium, chlorine and calcium chloride.
ability to conduct electricity
substance
relative melting
point
when solid
when molten
calcium
high
good
good
chlorine
low
poor
poor
calcium chloride
high
poor
good
Figure 7
Explain, in terms of bonding and structure, why the properties of the product,
calcium chloride, are different from the properties of the reactants, calcium
and chlorine.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Calcium chloride can be prepared by the reaction of calcium with chlorine gas.
Figure 7 shows some...
Questions


Mathematics, 11.10.2019 04:50




English, 11.10.2019 04:50

Biology, 11.10.2019 04:50

World Languages, 11.10.2019 04:50

Biology, 11.10.2019 04:50



Physics, 11.10.2019 04:50

English, 11.10.2019 04:50



Mathematics, 11.10.2019 04:50

English, 11.10.2019 04:50

Mathematics, 11.10.2019 04:50

Mathematics, 11.10.2019 04:50



