
Chemistry, 11.03.2021 14:40 chrisgonzv1219
Determine the molecular and empirical formulas for the substance shown in the ball-and-stick model below.
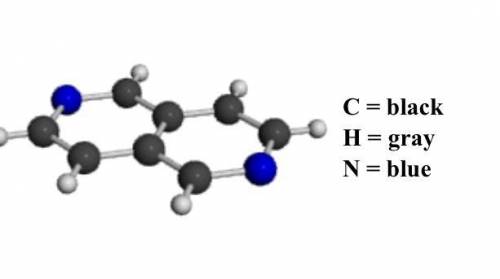

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
Determine the molecular and empirical formulas for the substance shown in the ball-and-stick model b...
Questions

History, 18.03.2021 14:00

Mathematics, 18.03.2021 14:00

Mathematics, 18.03.2021 14:00

Mathematics, 18.03.2021 14:00

Mathematics, 18.03.2021 14:00

Mathematics, 18.03.2021 14:00

Mathematics, 18.03.2021 14:00

Geography, 18.03.2021 14:00


Arts, 18.03.2021 14:00

Mathematics, 18.03.2021 14:00

History, 18.03.2021 14:00

Computers and Technology, 18.03.2021 14:00


English, 18.03.2021 14:00


History, 18.03.2021 14:00

Chemistry, 18.03.2021 14:00

History, 18.03.2021 14:00

History, 18.03.2021 14:00



