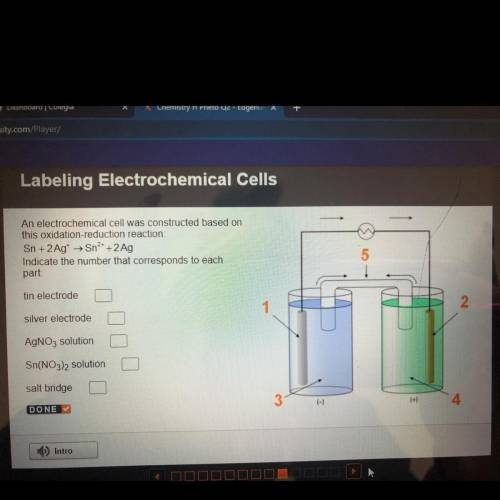
An electrochemical cell was constructed based on
this oxidation-reduction reaction:
Sn + 2 Ag...

Chemistry, 11.03.2021 18:00 emacwhaleng
An electrochemical cell was constructed based on
this oxidation-reduction reaction:
Sn + 2 Ag+ + Sn2+ + 2 Ag
Indicate the number that corresponds to each
part:
tin electrode
silver electrode
AgNO3 solution
Sn(NO3)2 solution
salt bridge

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3
You know the right answer?
Questions

Mathematics, 27.01.2021 21:20




Mathematics, 27.01.2021 21:20

English, 27.01.2021 21:20

Health, 27.01.2021 21:20

Chemistry, 27.01.2021 21:20

Mathematics, 27.01.2021 21:20


History, 27.01.2021 21:20

Mathematics, 27.01.2021 21:20


Mathematics, 27.01.2021 21:20

Arts, 27.01.2021 21:20

Mathematics, 27.01.2021 21:20


Mathematics, 27.01.2021 21:20




